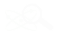Three Quick Tips To Increase your pH sensor selection Skills
Recommended practices to extend your pH sensor lifetime 5/5: Sensor Selection
- Miranda de Bruijn
The lifetime of a pH sensor has a significant impact on the total costs of ownership and performance of the entire pH measuring loop. Improving four key areas will lower costs and optimize process control and overall plant efficiency. These four key areas are:
- Maintenance & Storage,
- Calibration,
- Engineering Skill-set
- Sensor Selection.
Sensor Selection
What if the lifetime of your pH sensor is very short: only 2-3 weeks ? Besides that, you need to calibrate it every day, and to make it even worse: clean it several times a day. Something definitely is going wrong! And this situation will most likely influence your pH sensor lifetime, decrease reliable process control and efficiency.
In this blog you will learn what how to optimize the lifetime of your pH sensor by looking into: proper sensor selection.
Selecting the correct pH sensor for each application plays an important role in optimizing the pH sensor lifetime. It is important to know that selection will become easier once you understand two things: the characteristics of the two pH sensor basic parts: pH sensor glass (1) and reference sensors (2), and finally, the effects of temperature and pressure (3).
- PH glass characteristics
First, let’s have a look at standard pH glass. The surface of the glass of a pH sensor has pores caused by the structure of the glass. These tiny pockets are perfect for H+ ions, which get stuck in them. Or as the industry calls it ‘the sensor is selective for H+ ions’ (see figure 1).
For this reason “Ion Selective Electrodes” (ISE) exist i.e.: Sodium or Chloride ion selective electrodes.
|
|
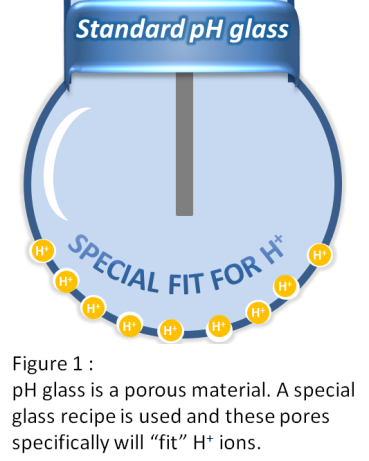
You might be wondering “Why is the above important to know ?” Let’s look at an example: in high alkaline applications (>pH12) the H+ ion concentration is very low. This is often done by dosing with Sodium Hydroxide (NaOH). This means that the Na+ concentration is high. The Na+ ions will block these H+ “pores” in the structure of the glass. Because these pores now have a Na+ instead of an H+ ion it will still see it as an H+ ion and register a lower pH than that it is in reality.
This is often referred to as the “Sodium Error” and occurs above a pH of 12. The reading of the measurement will be typically 0.17 pH lower than it is in reality (see figure 2). This is true for all pH sensors; not just the ones the company I work for (Yokogawa) makes. It is the nature of pH glass and measuring principle.
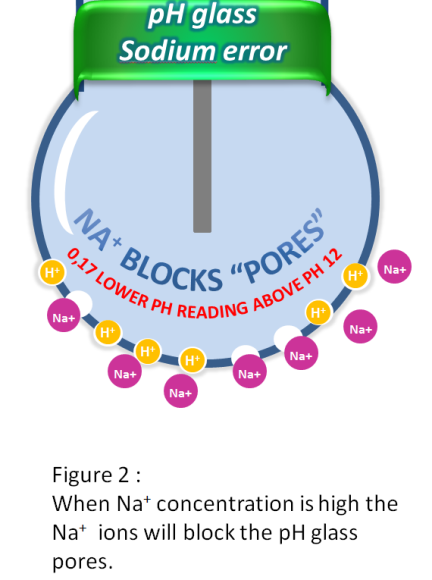
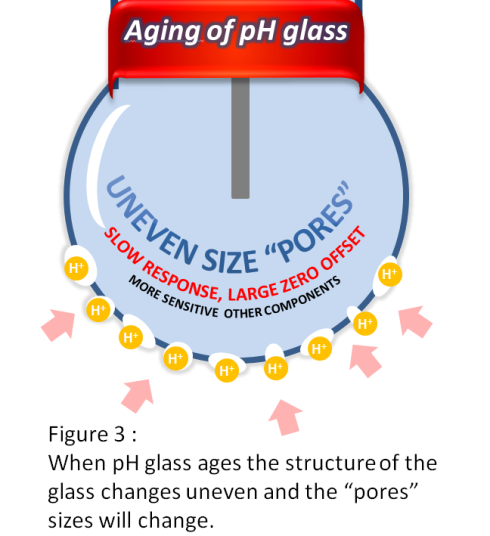
Another factor is aging of the pH glass itself. This will cause the structure of the glass to become uneven and will dissolve in the process over time. The sensor will then become sensitive not only to H+ ions, but also to other ions (see figure 3).
|
|
Knowing the glass characteristics of each sensor type will help to select the correct one for your application and prevent unnecessary replacements of sensors in the future.
One of the most common questions we get is : “How will I know when to replace my pH sensor ?” The answer I normally give is usually quite straightforward. During the calibration of the pH sensor the sensor will probably start to respond slowly to pH changes and in addition the zero offset will increase. This is a good indication that it is time to replace the pH sensor.
Another common question I received is : “What are the suggested limited for the zero offset ?”. If you consider that ± 60 mV corresponds to a difference in a 1 pH unit shift at 25°C, then if your sensor showed a zero offset of 30 mV, the analyser would have to adjust for 0.5 pH difference. In most cases a maximum difference of between 30-40mV is allowed before the sensor needs to be changed (this is true for pH only or combined sensors).
Note that most analysers will warn you after a true two point calibration is completed when the zero offset it out of limits. It is recommended to check the limits in the analyzer, because some instruments are defaulted to alarm at 120mV and that is 2 pH units and not good practice.
|
|
For the company I work for (Yokogawa) there are two types of pH glass sensors available. In order to minimize aging and corrosion of the glass it is important to select the correct type. This will also help to extend the pH sensor lifetime.
- Reference sensor characteristics
The Reference sensor completes the electrical circuit of the measuring loop and provides a stable reference potential. Between 70-80% of the pH sensor failures are generally caused by an issue with the reference junction. Selecting the correct type of reference junction is therefore an important step and will significantly increase the lifetime of the pH sensor. 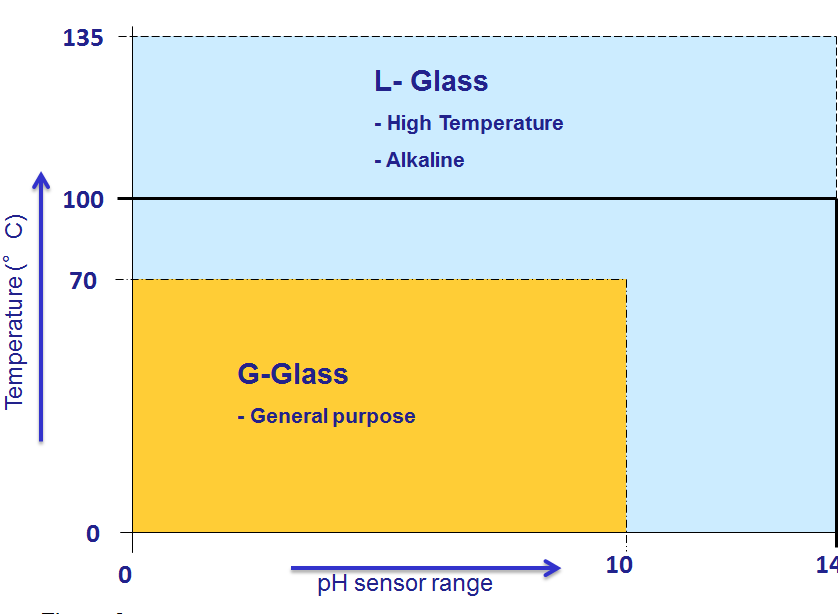
The purpose of the junction is to maintain contact between the reference system in the electrode and the process liquid.
Selecting the correct junction ensures the process liquid does not penetrate into the electrode. This is something to be avoided as it causes poisoning, and it might even create an unstable liquid junction potential.
The selection of the correct type of reference electrode junction, depends on the process conditions under which this electrode has to do its job. There are several different types of junctions. Four of the most commonly available junctions are :
- Ceramic
- PTFE
- Sleeve
- Salt sensitive glass
Each of these junction materials have their own particular advantages and disadvantages.
Ceramic junctions have very large pores. This means the ceramic junction has to be small. The added advantage is that the electrolyte inside the sensor will not deplete very fast in applications for example with a high salt content. The downside is that in dirty applications it is easily blocked, unless a flowing type sensor is used (cleans itself). (See figure 4)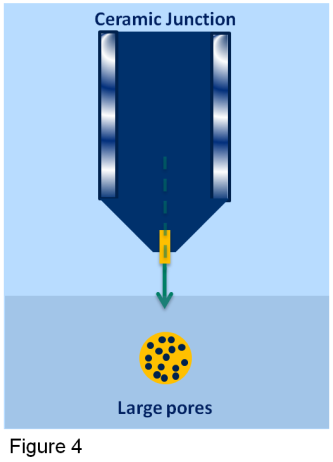
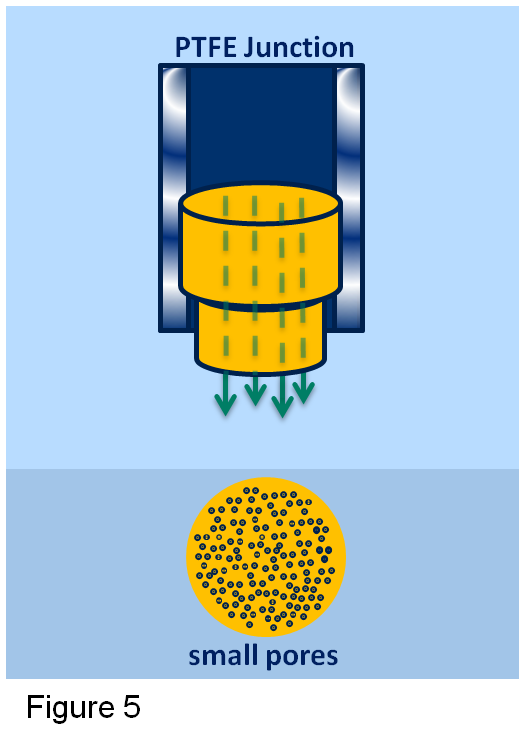
PTFE is well known for its fouling resistant properties. In applications where there is a lot of fouling or scaling it will repel the dirt and keep the junction free. The downside of having a PTFE junction are small pores, which only allows a small flow out of the sensor. When measuring in solutions with a high concentration of salts, this low flow can generate a potential difference across the PTFE junction (see figure 5) generating an error in the pH measurement.
The Sleeve is a very special type of junction. There is one hole in the main glass tube. Around the hole the glass is made deliberately uneven by lightly sandblasting. This creates small pathways for the electrolyte to flow from the sensor. Over this hole another sandblasted glass tube is placed (the sleeve). This will ensure a steady small flow of electrolyte out of the sensor. This is especially important in applications where the conductivity is low. The glass sleeve can be lifted to clean the pathways, which is especially handy for very dirty applications (see figure 6).
Salt sensitive glass is used in applications where there is a high salt concentration. High concentrations always flow towards the lower concentration and as a result the process will slowly ingress into the reference sensor and poison it over time. When using a glass reference, there is no open connection to the process and the sensor will not be poisoned. The salt in the process will generate a potential difference across the glass which is proportional to the salt concentration. As long as the concentration is relatively stable (± 25%) this reference will eliminate the poisoning and help provide a stable measurement.
To Flow or not to Flow?
Another question that you might be asking yourself is : “When shall I select a flowing type reference sensor and when a non-flowing type ? “
The following two situations are the only times you would consider using a flowing reference:
- Heavy fouling/scaling
- The electrolyte flowing out of the sensor will keep the junction clean.
- Low conductivity
- Everything flows from high to low. High salt concentration in the sensor, low in the process. The electrolyte will flow fast out of the sensor.
- When using a non-flowing type sensor it will cause the sensor to deplete fast and fail.
- The effect of temperature and pressure
Finally, I would like to discuss the effects of temperature and pressure. One of the most effective steps to increase the pH sensor lifetime is to decrease the temperature. Each 10 degree C decrease will double the lifetime of the sensor.
At higher temperatures the pH sensitive glass will age quicker and for the reference portion, the pressure will increase inside the sensor and cause the electrolyte to deplete faster (see figure 6).
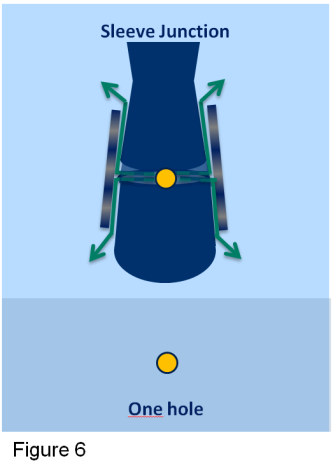
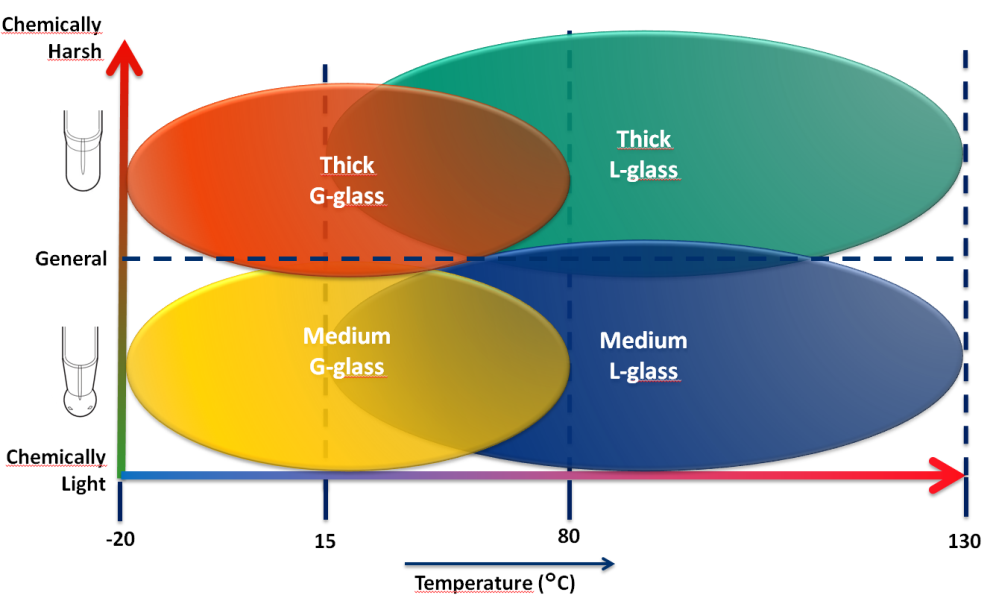
Figure 7 : Selection guide for Yokogawa pH sensitive glass
Fluctuating process pressure and temperatures will cause a pump effect inside the reference electrode sucking in the process liquid. This will significantly decrease the lifetime of the pH sensor.
Using a pH sensor with a bellow (always 300 mbar overpressure) inside the sensor will limit or even illuminate the negative effects of pressure and temperature fluctuations.
Summary
The next time you have to select a pH sensor for you application hopefully you can use this blog as a reference guide. I hope it will help you select the correct pH glass and reference type to improve your pH sensor lifetime and you can limit or even eliminate the effects of temperature and pressure on especially the reference sensor.
For more information on how to extend the lifetime of your pH sensor please check out our eBook here or through this link :
http://info.yokogawa.eu/acton/media/18463/ph-sensor-lifetime?sid=TV2:vDjdV5EAe
AnalyzeDetectNetwork.com